Data From:
Lin Huang, Keiko Kumura Ishii, Harmon Zuccola, Amy M. Gehring, Charles B. C. Hwang, James Hogle, and Donald M. Coen. 1999. "The enzymological basis for resistance of herpesvirus DNA polymerase mutants to acyclovir: Relationship to the structure of -like DNA polymerases." PNAS 1999 96: 447-452 (Pubmed)
Huang et al, The enzymological basis for resistance of herpesvirus DNA polymerase mutants to acyclovir: Relationship to the structure of -like DNA polymerases PNAS 1999. 96: 447-452
Here are supplemental data from this publication, i.e. the 'data not shown' within the paper.
Please click on image to zoom.
| a |
|
b |
|
c |
 |
|
 |
|
 |
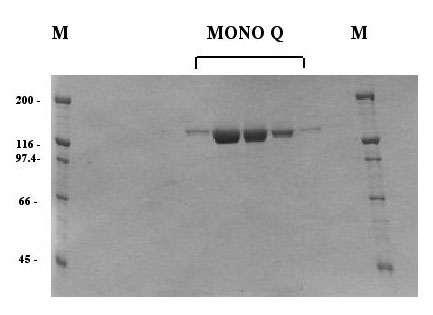
| Purification of HSV Pol. The figure shows a Coomassie blue-stained gel of fractions from the final purification step (Mono Q column) of wt Pol expressed in insect cells. The enzyme was purified to apparent homogeneity. |
|
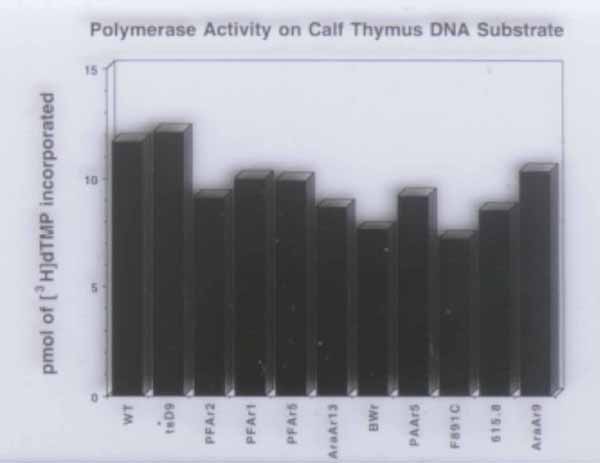
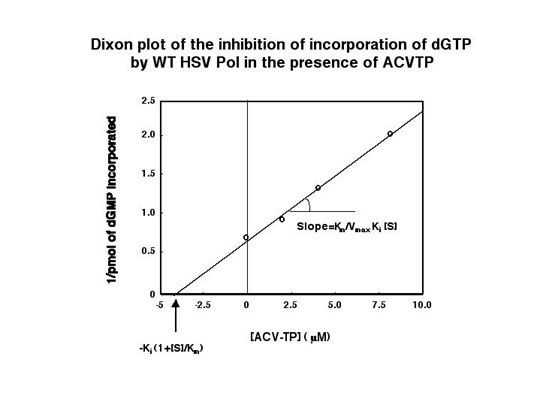
Specific activities of wt and mutant Pols using activated calf thymus DNA as primer-template and the 4 dNTPs as substrates and assayed as described by Weisshart and Knopf (Eur. J. Biochem. 174:707-716, 1988). All of the enzymes exhibited similar specific activities. Note that aside from the eight mutant Pols described in Table 1, two other mutant Pols (BWr and AraAr13) were also purified. These two mutant enzymes, however, incorporated ACV-TP too poorly to analyze by steady-state kinetic assays. |
|
|
| |
|
|
|
|
| d |
|
e |
|
f |
 |
|
 |
|
 |
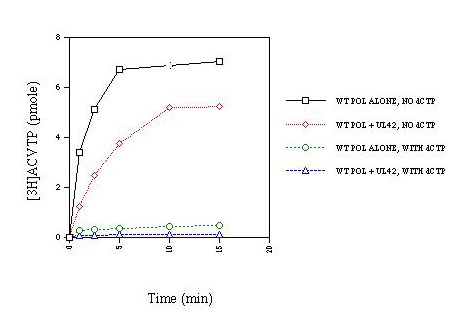
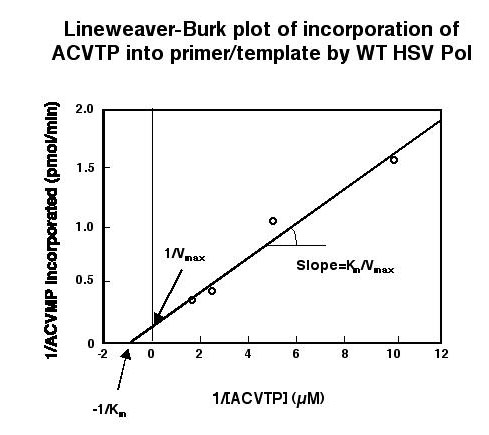
| Incorporation of ACV-TP into synthetic primer-template by Pol alone or Pol + UL42 under steady-state conditions (i.e., in primer-template excess). In the absence of dCTP (the dNTP that ordirnarily would be incorporated after ACV-TP if there were no chain termination), Pol + UL42 incorporated ACV-TP efficiently as previously reported (ref. 4). Pol alone incorporated ACV-TP at an even greater rate. With either Pol alone or Pol + UL42, the presence of dCTP greatly inhibited incorporation due to trapping of enzyme on primer-template, preventing dissociation and then binding to the next primer-template. |
|
|





